‘Mass-producing’ transfusion blood platelets
A group of research scientists have developed a microfluidic process capable of producing large quantities of blood platelets in several hours only. Their work opens up new pathways to in vitro production of platelets and stems from a collaboration between physicists and biologists (the Gulliver Lab., ESPCI Paris and the Inserm Institute and the start-up PlatOD). The results of the studies were published in Nature Scientific Reports and have led to registration of a joint patent claim by ESPCI, the CNRS, Inserm and PlatOD. Anne Le Goff, now a research scientist at the UTC-BMBI Lab relates the investigations in which she participated.
Blood platelets are mammal non-nucleated cells, only a few microns in diameter, absolutely necessary for blood to coagulate (clot). The demand for transfusion of platelets is increasing constantly, notably because of the multiplication of chemotherapy protocols and of bone marrow transplants. From a physiological point of view, platelets are formed by fragmentation of the cytoplasm of larger cells, the bone marrow megakaryocytes. We have known for a number of years now that the flood flow in capillary vessels irrigating the marrow plays an essential role in the formation of platelets. It was this discovery that motivated increased research activities in the field of microfluidics, to study the fragmentation of the megakaryocytes and blood platelet production. Most systems to date mime the crossing of the bone marrow by the megakaryocytes. And, although the initial observations were successful in showing how the cytoplasm fragments into platelets, the quantities of the latter were far too low to enable any valid biological characterization.
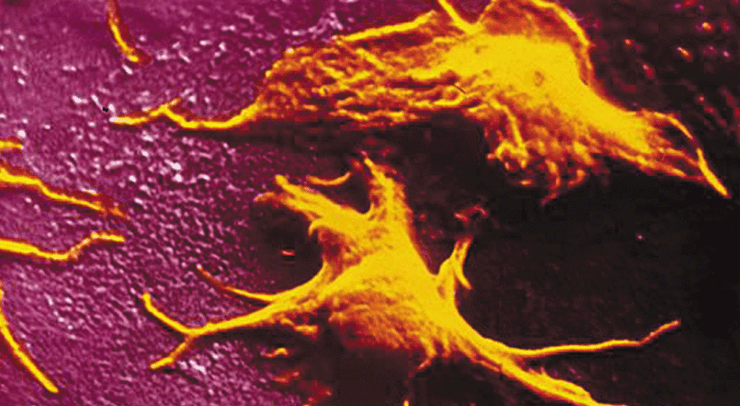
The scientists in our team chose a different approach, which was not limited to reproducing exactly the mechanisms taking place in the bone marrow. In the system they investigated, the megakaryocyte suspension was made to flow directly in a microfluidics chamber with a very large number of cross-strut pillars to which the cells adhere, while remaining subject to the hydrodynamic forces at play, the latter enhancing their elongation and fragmentation. What the scientist observed was a reorganization of the megakaryocyte cytoskeleton, to take on the shape of ‘pearl necklaces’ (cf. photo). The cytoplasm then subdivides, under the effect of the hydrodynamic forces and releases platelets into the perfusion stream on a sustained, regular basis. “Thanks to these hundreds of micro-pillar structures in our lab-on-chip, we were able — over a two-hour period — to produce a sufficient quantity of platelets, viz., enough to allow their characterization. We demonstrated that — as we had expected — the platelets were not activated as they left the bio-reactor chamber but remained sensitize to chemical activation which is what we need to have them fulfil a clotting function in the receiver’s blood-stream“, says Mathilde Reyssat, a research scientist at the Gulliver Lab, ESPCI, Paris.
This original set of results derives from a rich collaborative effort by physicists, biologists and medical practitioners. The academic research staff worked hand in hands with the engineers from the PlatOD start-up, created by Dominique Baruch, whose objective is to produce platelets “on demand” within the next few years.
This work is a first step towards large-scale blood platelet production in vitro and therefore towards new forms of transfusion protocol. There is still a long list of studies remaining to be carried out, such as animal in vivo coagulation tests and in the long-term, some clinical trials are expected, in just a few years’ time.




